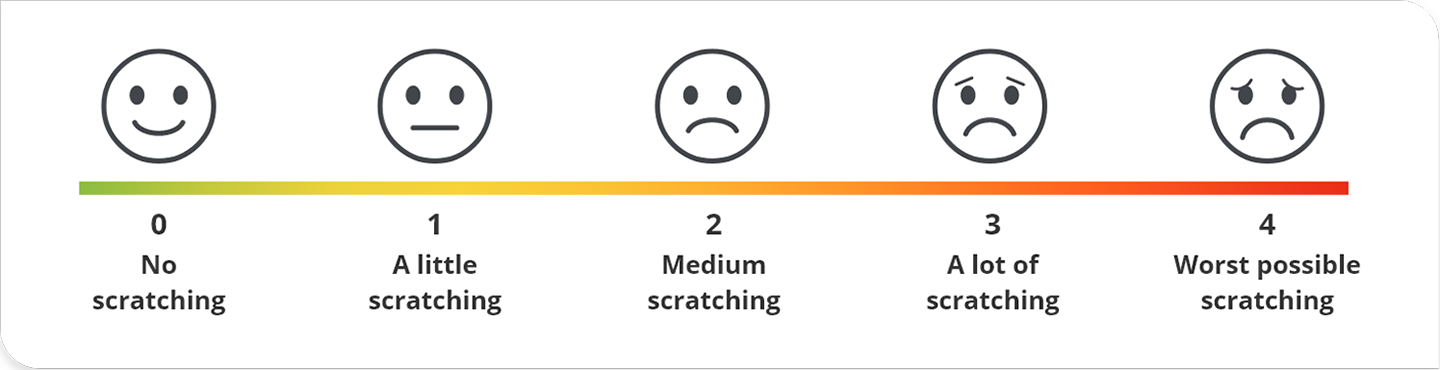
In the PEDFIC trials, cholestatic pruritus was measured using the PRUCISION™ ObsRO scale1,2
- A validated 5-point scale from 0 (“No scratching”) to 4 (“Worst possible scratching”)
- Scratching scores were recorded twice daily by an observer
- All patients had medium to severe cholestatic pruritus at baseline (ObsRO score ≥2)
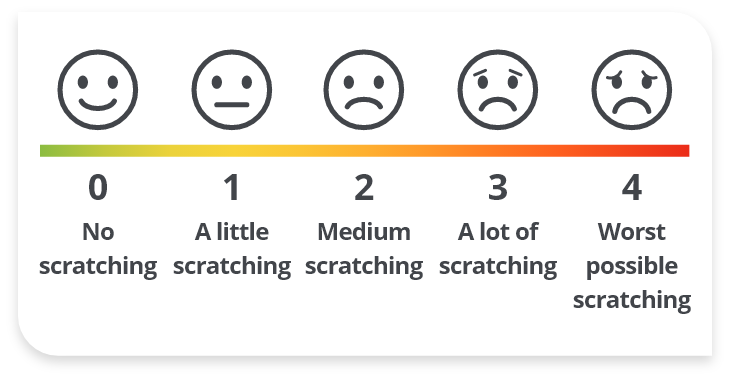
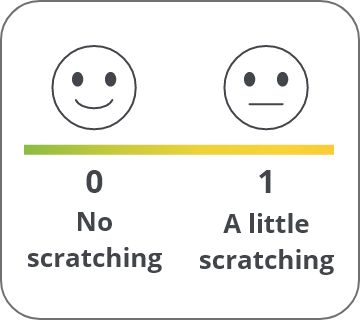
A reduction of ≥1 point is a meaningful score change in cholestatic pruritus2
Consider what even a small reduction in pruritus could mean for your patients with PFIC.
Primary endpoint: Change in cholestatic pruritus.
PEDFIC 2 is an ongoing open-label extension trial. Data shown are interim data.
Little to no scratching achieved with Bylvay1
At Week 241
More than
1 out of 3
pruritus assessments achieved a score of ≤1*

35% with Bylvay 120 mcg/kg/day (n=23)*
30% on Bylvay 120 mcg/kg/day (n=19)
vs 13% with placebo (n=20)
Through Week 483
More than
4 out of 10
pruritus assessments achieved a score of ≤1†


44% of Bylvay patients treated for 48 weeks (n=71)
66% and 84%
of patients experienced a ≥1-point improvement in pruritus at weeks 48 and 96, respectively—a clinically meaningful score change (n=71, n=19)2,4‡
*Mean difference vs placebo (95% CI): Bylvay 40 mcg/kg/day: 22.2 (4.7, 39.6); Bylvay 120 mcg/kg/day: 16.9 (-2.0, 35.7). N=62.
†Data are the combination of weeks 45-48 and weeks 37-48. Subjects are counted only once. All patients were transitioned to Bylvay 120 mcg/kg/day at 24 weeks. N=111.5
‡Reduction from baseline pruritus score at last available assessment up to weeks 85-96. This pooled analysis covers the period from the first-ever dose of odevixibat in PEDFIC 1 or PEDFIC 2 through January 31, 2022. N=111.
*Mean difference vs placebo (95% CI): Bylvay 40 mcg/kg/
day: 22.2 (4.7, 39.6); Bylvay 120 mcg/kg/day: 16.9 (-2.0,
35.7). N=62.
†Data are the combination of weeks 45-48 and weeks
37-48. Subjects are counted only once. All patients were
transitioned to Bylvay 120 mcg/kg/day at 24 weeks.
N=111.5
‡Reduction from baseline pruritus score at last available assessment up to weeks 85-96. This pooled analysis covers the period from the first-ever dose of odevixibat in PEDFIC 1 or PEDFIC 2 through January 31, 2022. N=111.
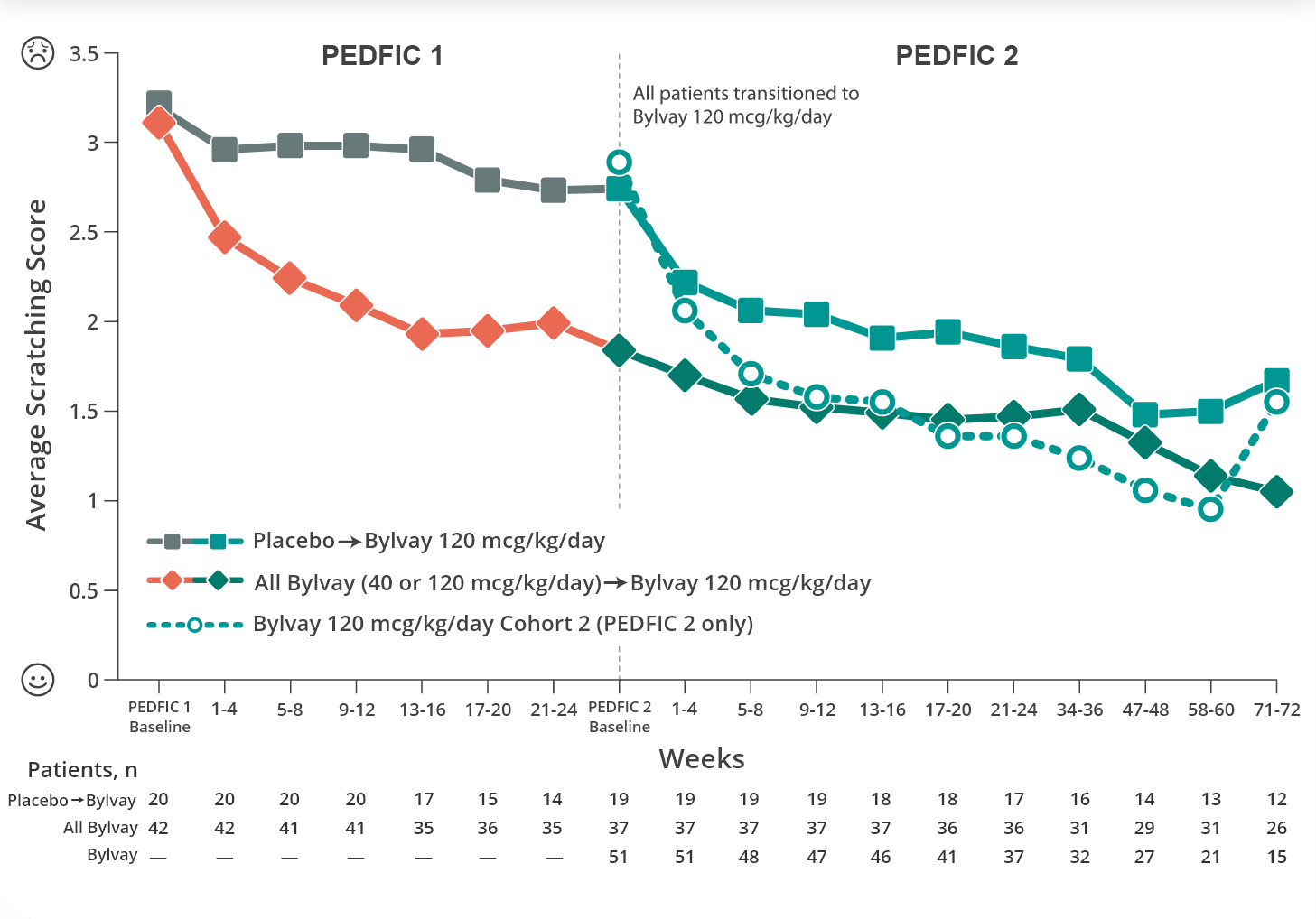
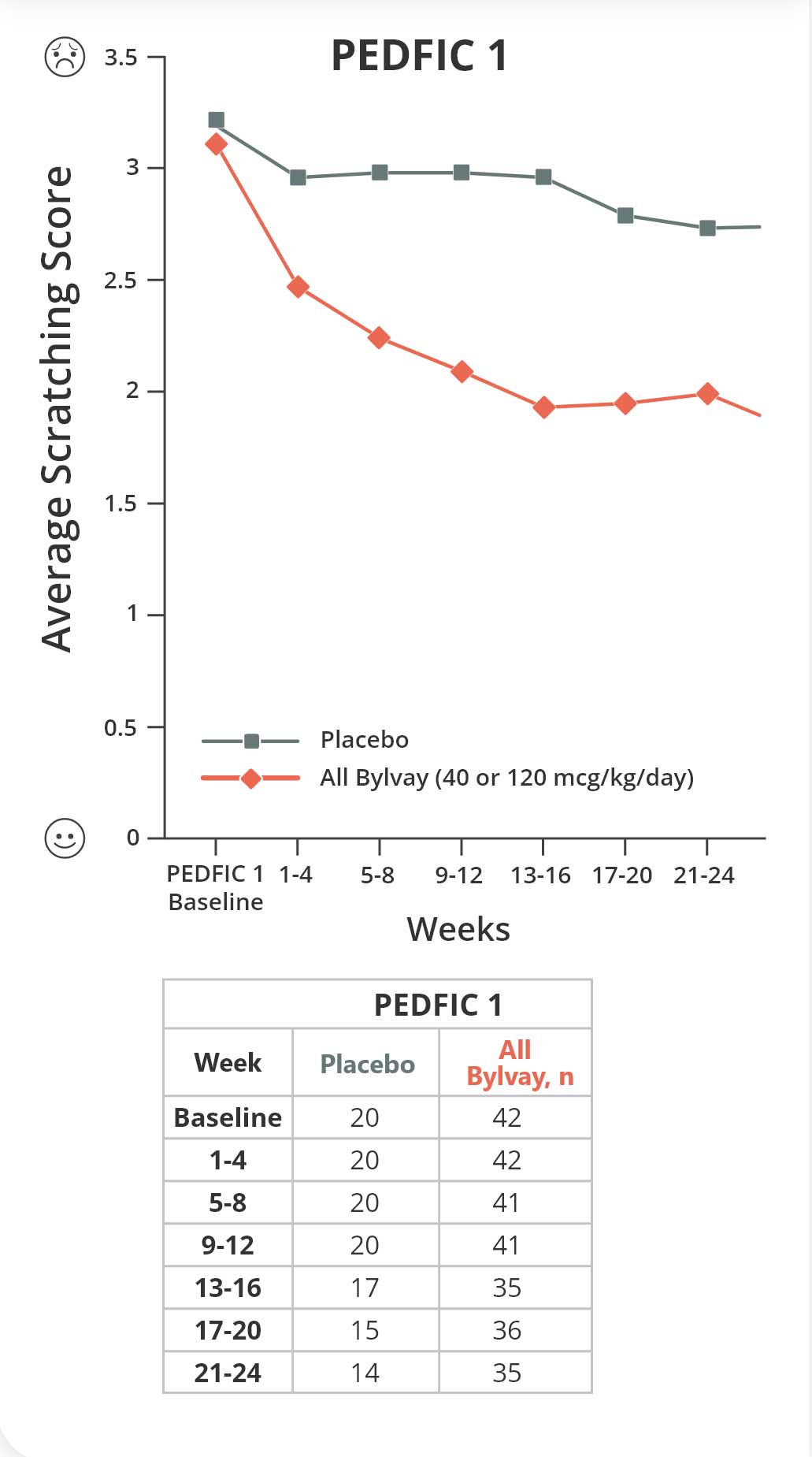
Bylvay reduced cholestatic pruritus early and over time6
Change in Scratching Score: PEDFIC 1 Through PEDFIC 2 Week 726
Change in Scratching Score: PEDFIC 1
Change in Scratching Score: PEDFIC 2 Week 726
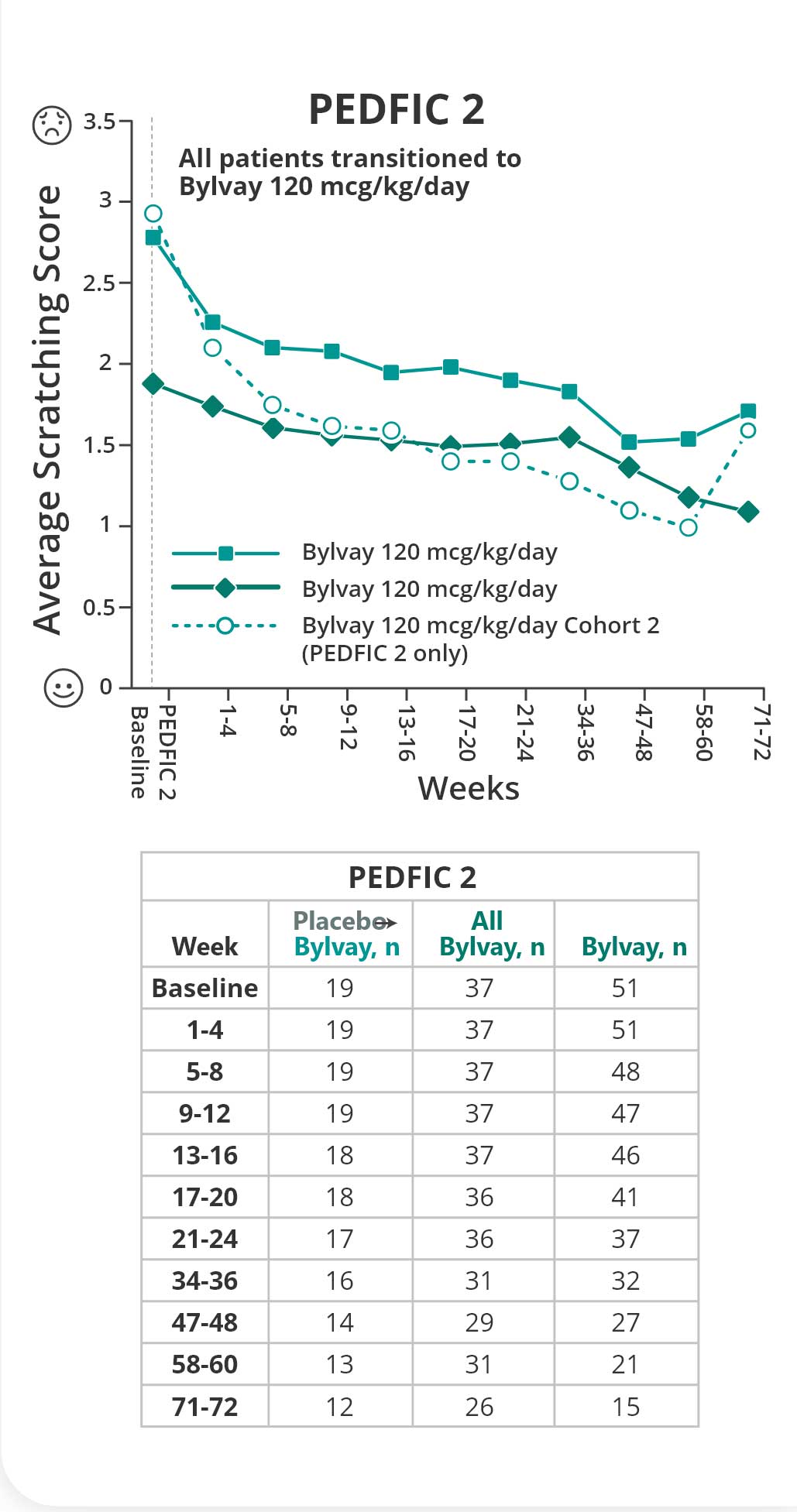
Reductions in cholestatic pruritus were seen as early as week 4 and sustained through week 966
Primary endpoint: Change in cholestatic pruritus as measured by proportion of positive pruritus assessments.
PEDFIC 2 is an ongoing open-label extension trial. Data shown are interim data.1,7,8
Learn how Bylvay impacted sleep in patients with PFIC
ObsRO=observer-reported outcomes; PFIC=progressive familial intrahepatic cholestasis.
References:
- Bylvay Prescribing Information. Boston, MA: Albireo Pharma, Inc.; 2023.
- Gwaltney C, Ivanescu C, Karlsson L, Warholic N, Kjems L, Horn P. Validation of the PRUCISION instruments in pediatric patients with progressive familial intrahepatic cholestasis. Adv Ther. 2022;39:5105-5125.
- Data on file A4250-ISE_T112_Pru Score Prured. November 10, 2022. Boston, MA: Albireo Pharma, Inc.
- Data on file A4250-ISE_T132_Pru Chg Prured. November 14, 2022. Boston, MA: Albireo Pharma, Inc.
- Data on file A4250-008. Boston, MA: Albireo Pharma, Inc.
- Data on file PEDFIC 1 and 2 Figure Pru and sBA. 2022. Boston, MA: Albireo Pharma, Inc.
- Thompson RJ, Arnell H, Artan R, et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7:830-842.
- ClinicalTrials.gov. An open-label extension study to evaluate long-term efficacy and safety of A4250 in children with progressive familial intrahepatic cholestasis types 1 and 2 (PEDFIC 2). NCT03659916. Updated October 12, 2022. Accessed April 23, 2023.

